TS Manager provides a validation system for autoclaves, compatible with FDA 21 CFR Part 11 regulations.
A Complete System for Thermal Validation TS Manager is a complete solution developed by Tecnosoft for all who need to monitor temperature in autoclaves, pasteurisers and other high temperature processes.
TS Manager is compatible with FDA 21 CFR Part 11 regulations, and may be used with all Tecnosoft devices including the PressureDisk for temperature and pressure monitoring. All of them can work up to 140°C, resist at least up to 6 bar of pressure and can be used to map the autoclave and check if it is working correclty. Designed with professional validators in mind, TS Manager makes it easy to track validations carried out at multiple sites and by different engineers.
The Software
The TS Manager software has been developed with two main ideas: being 21 CFR Part 11 compatible and being a “must-have” resource for all validators of autoclaves, incubators, pasteurisers and eneral equipment in the healthcare and food industry. It has all the features idicated by FDA regulations and other features that let the user manage validations for different Customers. Its intuitive interface puts in your hands a powerful tool but it is very easy to use. Create your database of Customers and Instruments to validate, create validation profiles to analyse the processes, start
your loggers and then download them to check all data collected.
Starting a monitoring mission is quick and simple: put the logger in the DiskInterface already connected via USB and just press Start. The software will automatically recognize the type of logger connected and will show its status. Proceed and choose the profile to use to analyse your data. Profiles are set of parameters such as acquisition rate, start delay, kind of analysis (F0, Overkilling, PU etc.). Here you can assign also a Customer and process you are going to validate (autoclave, pasteuriser etc.). In the next step the logger will be programmed and you can assign also the use you are currently making of it (validation, test, production control etc.).
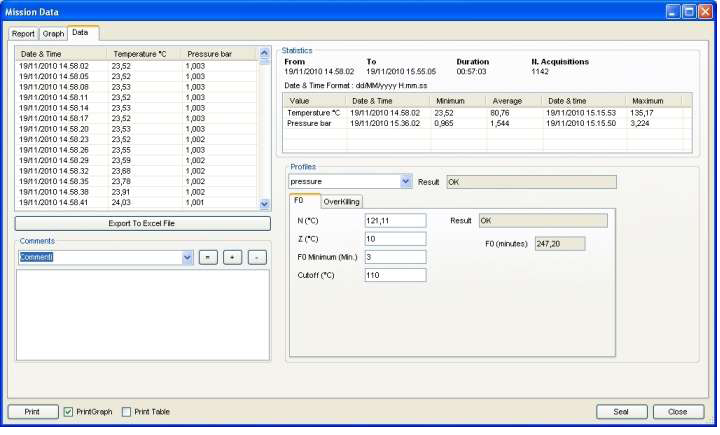
Data Analysis
TS Manager offers you several tools to analyse your data. A complete HTML report is displayed with all the details from your process: type of analysis, result, logger used, user who programmed and downloaded it, all data acquired. The Graph has zoom feature, vertical and horizontal markers to set points for analysis both on temperature and pressure (you can define a portion of graph to analyse and calculate span between max and min, time span etc.). You can also customize the grid of the graph easily. The Data tab allows for a quick reading of all the data, statistics such as max, min and average and the profile analysis, like F0 calculation and result according to expected values. You can also export data to Excel.
Printed Report
TS Manager lets you print complete reports of your
processes, with header image of your company and all data to track the mission. You can choose to print graph, data table. Reports are printed according to regulations and can be signed on each page by the validator or the user.
TS Manager Specifications
Operating systems | Windows XP / Vista / 7 |
Data management | Database SQL client/server installation or stand-alone, data saved per mission (start and stop of acquisitions); available archives: mission templates (profiles), missions, customers and instruments (for validators), users (with login, password, permissions and validity period), stations (PCs where the software is installed), devices (list of all devices connected and used for validation), activity log (registry |
Data presentation | HTML report (exportable), graph with zoom and vertical/horizontal markers, table with statistics (max, min, average) and analysis (F0, PU, overkilling), export into Excel, report printing |
CFR21 Part 11 compliance | FDA 21 CFR Part 11 compatible: users have login, password and permissions, data cannot be edited, devices cannot be tampered, database hacks checking feature for auditors, Audit option, Sealing feature for analysis on data (avoid further changes), all actions recorded in the log, printed reports |
Languages | English, Italian |
If you would like more information, please contact us to "Get On TRAQuE"